As organisms age, they accumulate cells that no longer function properly but have managed to escape cellular death. These “zombie cells” wreak havoc by pumping out inflammatory signals.
Studies show that if you can remove these cells in mice, they have longer life and health spans. Yet despite decades of study, this cellular state is not fully understood in culture and even much less so during human aging. This state, termed replicative senescence, has become a prominent model for researching cellular aging in vitro.
Revisiting Len Hayflick’s renowned 1960s study
In recent years, Calico has revisited the original observation of replicative senescence in fetal lung cells by Len Hayflick, PhD, professor of anatomy at UCSF, who conducted the work during the 1960s at Wistar Institute in Philadelphia. The Calico study has now delivered a high-resolution molecular description of senescent cells. It has also revealed new insights to help scientists better understand senescent cells and how to target them.
The findings have impressed Len. “Of the more than 13,500 research papers that cite my finding of mortal senescent normal human cells as distinguished from immortal cancer cells, most are descriptive,” says Len. “This paper is one of the few that reports the results of innovative research and fundamental findings.”
Shedding light on senescent cells and how they work
To shed light on what exactly senescent cells are and how they work, Michelle Chan and Dave Hendrickson led a Calico team to generate a complex data set including RNA-seq, single cell RNA-seq, proteomics, metabolomics and ATAC-seq. “They were able to reveal new facts about changes that occur at the molecular level in normal human cells both before and during the senescent state,” explains Len.
Among many insights, the team observed that senescent cells share a lot of similarities with myofibroblasts, which are wound-healing cells found in connective tissue that secrete collagen proteins. “This is significant because we know a lot about wound healing and myofibroblasts; filing senescent cells in that category opens up new ways to think about it,” says Dave, adding, “The next step will be to take what was learned, apply it to in vivo models and explore ways to target senescent cells or alter their behavior.”
Read the full study published in eLife
Read more about the significance of the paper results in the eLife Insight article Senescence: A gradual path to mortality (authored by Yang N, Sen P).
Images below
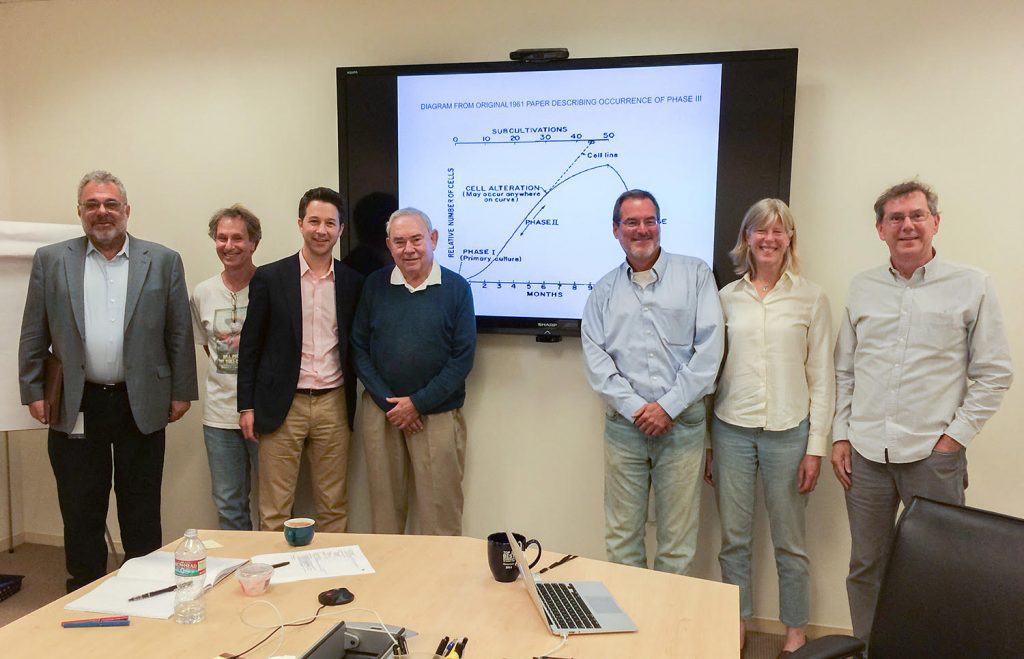
On June 26, 2014, Len Hayflick gave a seminar on his research at Calico. Pictured from left to right: David Botstein, Bob Cohen, Jonathan Lewis, Len Hayflick, Hal Barron, Cynthia Kenyon, Art Levinson.
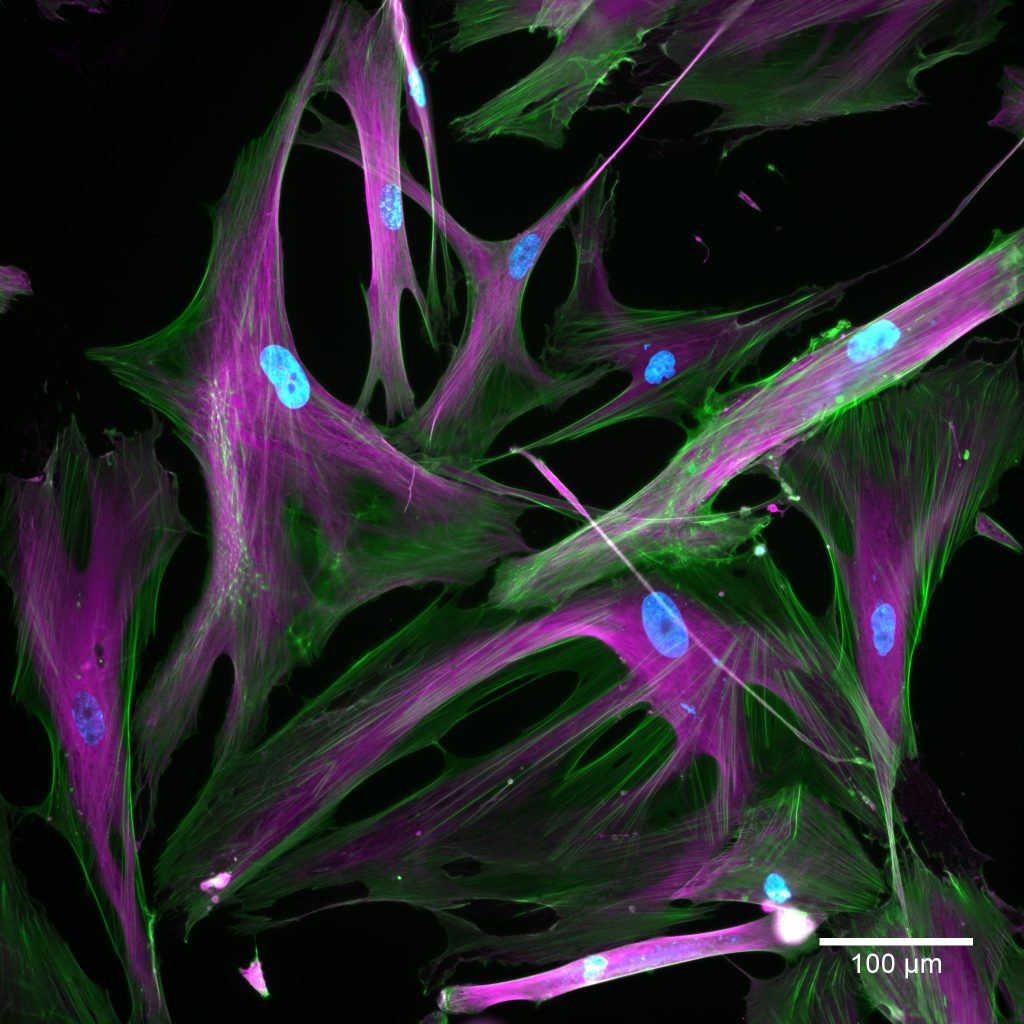
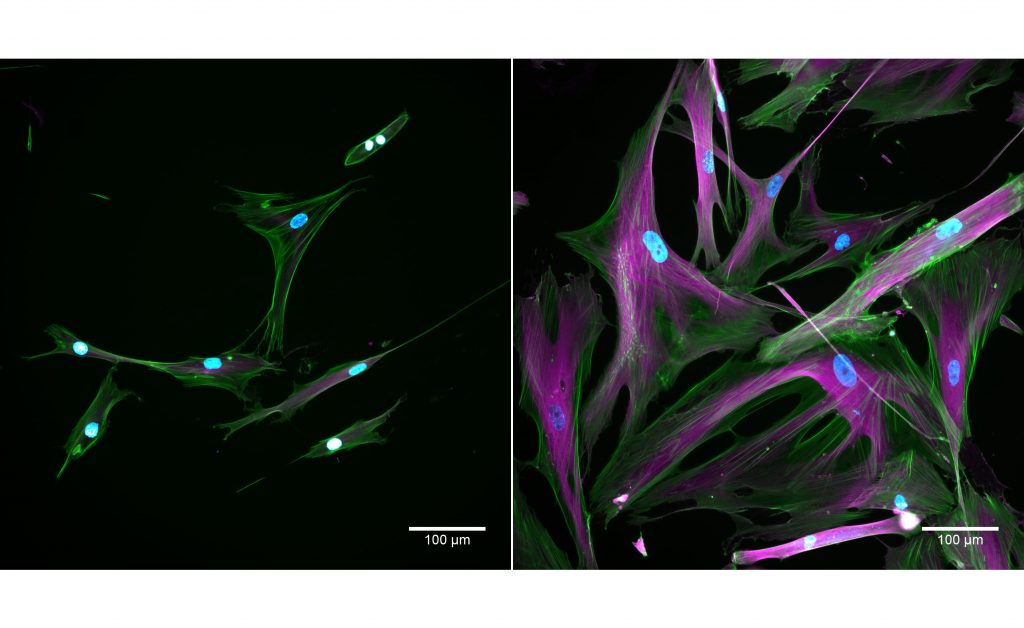
Cells taken using a fluorescent microscope. Normal cells appear on the left and senescent cells appear on the right. The stains show nuclei in blue; actin in green; tubulin in magenta. Credit: Kayley Hake and Nelda Yi
"Of the more than 13,500 research papers that cite my finding of mortal senescent normal human cells as distinguished from immortal cancer cells, most are descriptive. This paper is one of the few that reports the results of innovative research and fundamental findings."